



業務咨詢
中國:
Email: marketing@medicilon.com.cn
業務咨詢專線:400-780-8018
(僅限服務咨詢,其他事宜請撥打川沙總部電話)
川沙總部電話: +86 (21) 5859-1500
海外:
+1(781)535-1428(U.S.)
0044 7790 816 954 (Europe)
Email:marketing@medicilon.com





The cyclopropyl ring has highly strained C-C bonds with enhanced π-character and stronger C-H bonds than those in other alkanes. These features make cyclopropane not only a highly versatile synthetic intermediate but also a valuable structural motif both in natural products and commercially available drugs for enhancing the pharmaceutical properties. Currently, the most reliable and frequently used method of constructing cyclopropane units involves alkene cyclopropanation using transition metal carbene species, which are usually generated from α-diazocarbonyl compounds. Thus, the development of a green and safe protocol is still highly warranted without the use of potentially hazardous diazo compounds and transition metal catalysts.
Organic electrochemistry has drawn great interest as a green synthetic tool. It can promote dehydrogenative processes through H2 evolution without the need of chemical oxidants. A recent report from Xu group (J. Am. Chem. Soc. 2022, 144, 2343.) indicated an electrocatalytic radical-polar crossover process. Various methylene substrates, in a sequential manner, is oxidized to a carbon radical which is cyclized and cross-coupled with the radical cation of the phenothiazine-based catalyst. Finally, it is converted to the cyclopropane product via intramolecular nucleophilic substitution.
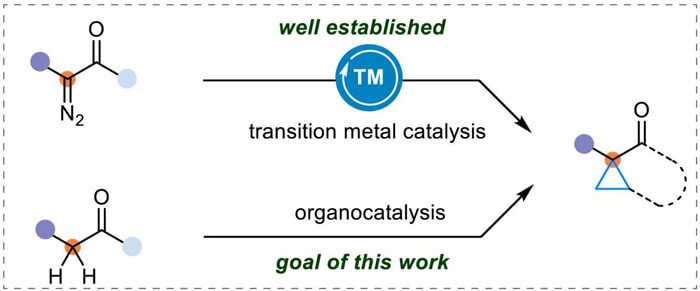
Common approach for cyclopropane synthesis and goal of this work
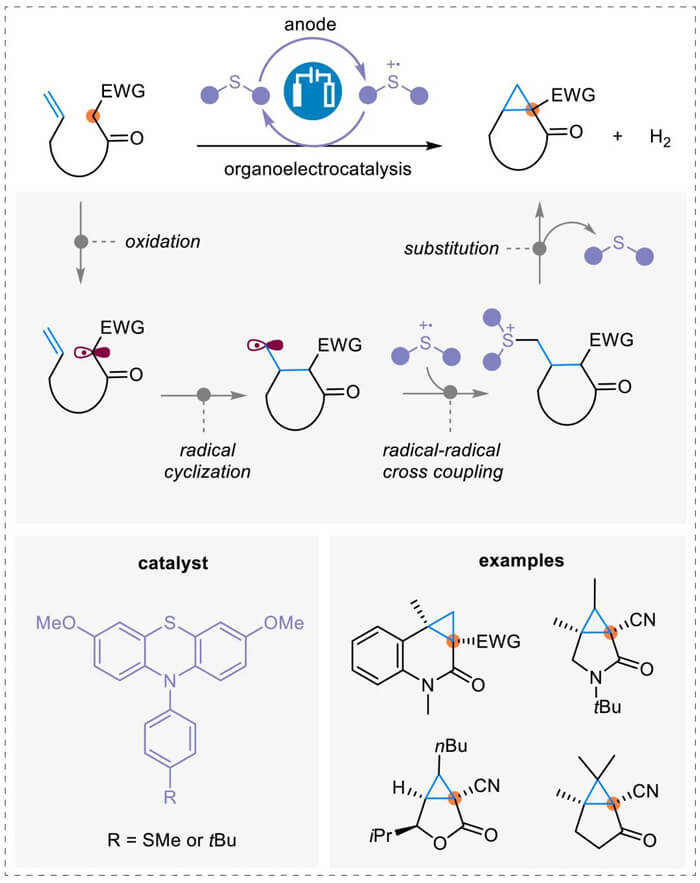
Electrocatalytic cyclopropanation of active methylene compounds
The authors used alkene-tethered α-cyanoamide 1 as the substrate to optimize the reaction conditions of the electrocatalytic cyclopropanation reaction. They found the desired cyclopropane-fused quinolinone 2 was obtained in a 96% yield by running the constant current (7.5 mA) electrolysis in a simple undivided cell (a Schlenk tube) in 2,2,2-trifluoroethanol (TFE) at 55 °C and using N-phenyl phenothiazine PT-1 (Ep/2 = 0.34 V versus the saturated calomel electrode (SCE)) as the catalyst and a low electrolyte loading (0.1 equiv, Et4NBF4) (entry 1). No additional acidic or basic additives were needed. Control experiments showed that electricity, organic catalyst, heating and N2protection were necessary. Electrolyte can be omitted at the expense of increased cell potential. Oxidation potentials ranging from 0.30 to 0.55 V versus the SCE were also found to be suitable catalysts (entries 7-9). Alternative solvents, such as MeOH or TFE/MeCN (1/1) can also be used.
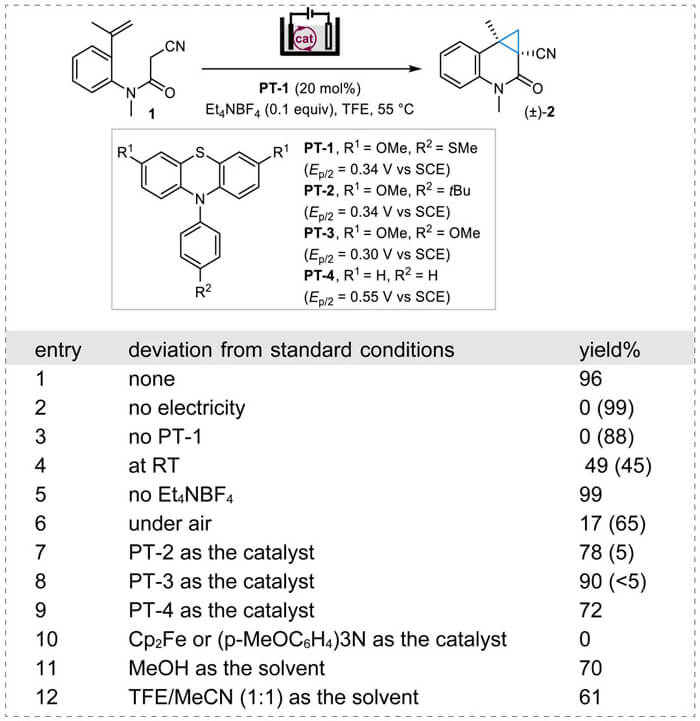
Optimization of Reaction Conditions
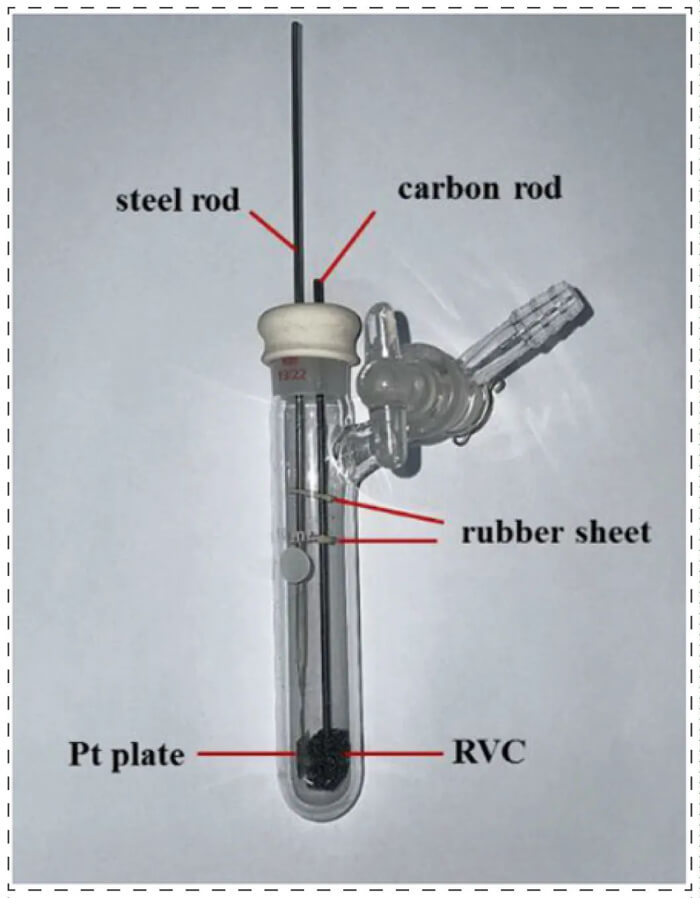
Reaction setup for small scale electrocatalytic reaction
The substrate scope for cyclopropane-fused quinolinones was investigated: mono-, di-, and trisubstituted acyclicalkenes, cyclic alkenes, different substitutions on benzo moiety, various N-protection groups and active methylene compounds were found to give desired cyclopropyl derivatives in good to moderate yield. The substrate scope for cyclopropane-fused lactams, lactones, and cyclic ketones was also investigated. During this study, they found that PT-2 and higher temperature were preferred for the preparation of compound from 30 to 49. Highly substituted cyclopropyl ring (51) was also obtained with moderate yield without the influence of steric effect.
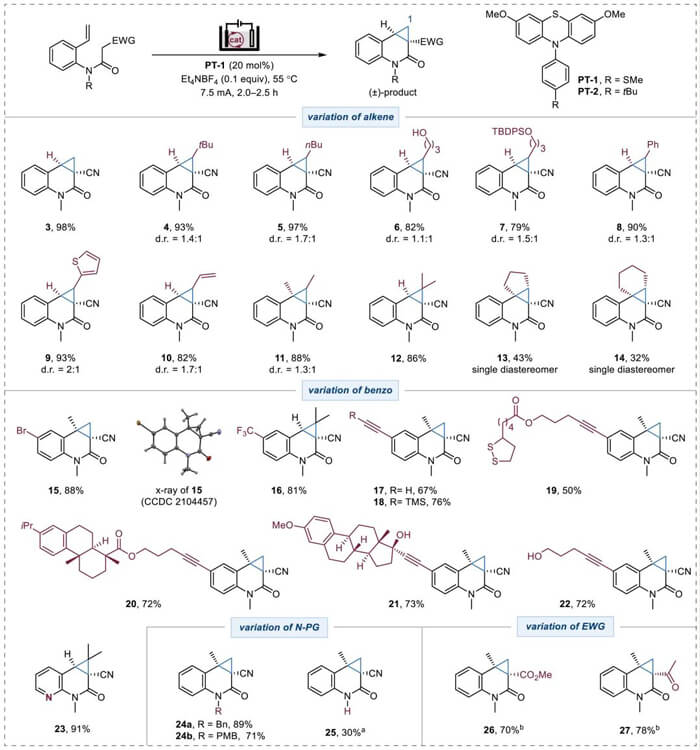
Substrate scope for cyclopropane-fused quinolinones

Substrate scope for cyclopropane-fused lactams, lactones, and cyclic ketones
After detailed mechanistic studies, the Xu group proposed a mechanism for the electrocatalytic cyclopropanation. PT-1 was oxidized to radical cation [PT-1]?+first. Then it oxidized the methylene substrate 70 to form electrophilic carbon radical 71. This radical first underwent the intramolecular cyclization to form alkyl radical 72, then formed sulfonium 73 through radical-radical coupling with [PT-1]?. Finally, CF3CH2O-, which was generated in situcontinuously at the cathode, triggered a nucleophilic substitution reaction to produce the final cyclopropanation product 74 and the regenerated PT-1.
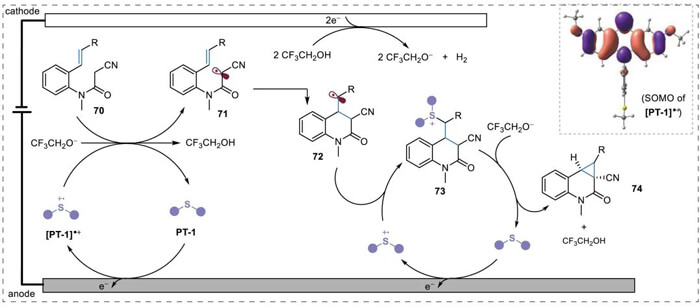
Mechanistic proposal
To evaluate the scalability of this methodology, the authors performed the organo electro catalytic reaction in a beaker-type cell with one or two RVC (100 PPI, 5.0 cm x 5.0 cm x 1.2 cm) anode, a Pt plate cathode (5.0 cm x 5.0 cm x 0.1 cm), and a constant current (187.5-375.0 mA) on multigram scale. It can be scaled up to more than 20 g with high yield. They found that the loadings of the electrolyte (Et4NBF4) for the decagram-scale reactions can further be reduced to 4-5 mol %.

Scale-up electro-catalysis to make cyclopropyl compounds
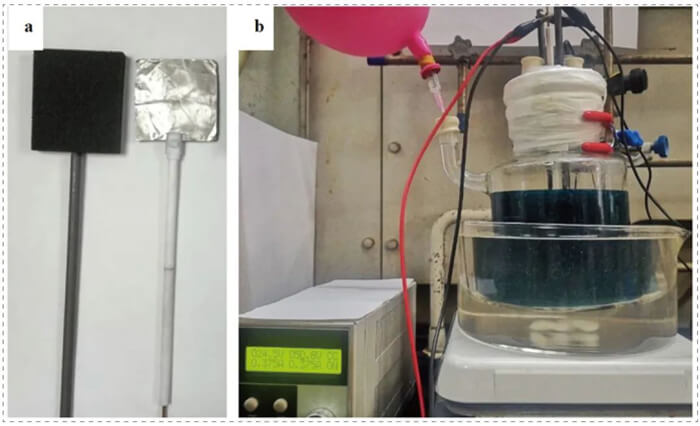
Reaction setup for scale up. a) Electrodes: RVC and Pt plate. b) Reaction setup.
In summary, the efficient and scalable intramolecular cyclopropanation of active methylene compounds via electrocatalysis with an organic catalyst can solve challenges from the transition metal catalyzed alkene cyclopropanation of functionalized compounds, such as α-diazocarbonyl compounds and increase operational safety.
Electroorganic synthesis has become an established, useful, and environmentally benign alternative to classic organic synthesis. Various electrochemical transformations have been well developed and utilized for industrial commodity chemical synthesis, such as the Kolbe reaction, the Simons fluorination process, the Monsanto adiponitrile process and so on. Medicilon has been vigorously developing new technologies over the years, integrating emerging methods of green chemistry into its services, using currently popular organic electrosynthesis to provide our customers with high-quality economic solutions.
? Highly experienced in different types of electrochemistry reactions.
? Full capacity for the synthesis of a variety of catalysts for organo electro catalytic reactions.
? Strong expertise in electrocatalytic chemistry.
References:
[1] Liang-Hua Jie, et al. Organoelectrocatalysis Enables Direct Cyclopropanation of Methylene Compounds. J Am Chem Soc. 2022 Feb 9;144(5):2343-2350. doi: 10.1021/jacs.1c12762.
[2] Cong Ma, et al. Recent advances in organic electrosynthesis employing transition metal complexes as electrocatalysts. Sci Bull (Beijing). 2021 Dec 15;66(23):2412-2429.doi: 10.1016/j.scib.2021.07.011.